Psychedelic drug
Pharmaceutical compound
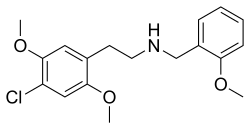
25C-NBOMe , also known as NBOMe-2C-C , 2C-C-NBOMe , or Cimbi-82 , is a psychedelic drug and derivative of the psychedelic phenethylamine 2C-C .[ 3] agonist of the 5-HT2A receptor ,[ 4] 11 Cradiolabelled form as a potential ligand for mapping the distribution of 5-HT2A receptors in the brain, using positron emission tomography (PET).[ 5] [ 6] scientific literature by 2010.[ 7] [ 5]
Use and effects
Blotter paper containing 25C-NBOMe 25C-NBOMe is extremely potent and the effects of the drug increase greatly within a small window of dosage adjustment. Overdose may occur at as little as double an average dose. With inaccurate dosing of street blotter paper , when mistaken for LSD, or when taken as a powder or liquid, this has resulted in multiple accidental deaths.[ 8]
One study has shown that 25C-NBOMe blotters have 'hotspots' of the drug and the dosage is not evenly applied over the surface of the paper, which could lead to overdose.[ 9] [ 10]
NBOMe-substituted compounds have a diminished absorption rate passing through mucous membranes, but generally remain inactive when taken orally. Buccal , sublingual or insufflated routes of administration are all viable options. Absorption rate buccally and sublingually can be increased when complexed with HPBCD complexing sugar, however the most efficient is nasal administration , which shortens the duration while increasing intensity, but has been attributed to several overdoses and deaths.[ 11]
Toxicity and harm potential
NBOMe compounds are often associated with life-threatening toxicity and death.[ 12] [ 13] [ 14] autonomic dysfunction remains prevalent with NBOMe compounds, with most individuals experiencing sympathomimetic toxicity such as vasoconstriction , hypertension and tachycardia in addition to hallucinations.[ 15] [ 16] [ 17] [ 18] [ 19] toxidrome include agitation or aggression , seizure , hyperthermia , diaphoresis , hypertonia , rhabdomyolysis , and death.[ 15] [ 19] [ 13] serotonin syndrome .[ 20] [ 14]
NBOMe and NBOHs are regularly sold as LSD in blotter papers,[ 13] [ 21] [ 15] [ 12] [ 12] [ 17] [ 15] self-harm and suicide under the influence of the substance.[ 22] [ 23] [ 15]
Given limited documentation of NBOMe consumption, the long-term effects of the substance remain unknown.
[ 15] NBOMe compounds are not active orally,
[ a] and are usually taken sublingually.
[ 25] : 3 When NBOMes are administered sublingually,
numbness of the tongue and mouth followed by a metallic chemical taste was observed, and researchers describe this physical side effect as one of the main discriminants between NBOMe compounds and LSD.
[ 26] [ 27] [ 28]
Neurotoxic and cardiotoxic actions
Many of the NBOMe compounds have high potency agonist activity at additional 5-HT receptors and prolonged activation of 5-HT2B can cause cardiac valvulopathy in high doses and chronic use.[ 13] [ 18] 2B receptors have been strongly implicated in causing drug-induced valvular heart disease .[ 29] [ 30] [ 31] adrenergic α1 receptor has been reported to contribute to the stimulant-type cardiovascular effects.[ 18]
In vitro studies, 25C-NBOMe has been shown to exhibit cytotoxicity on neuronal cell lines SH-SY5Y , PC12 , and SN471, and the compound was more potent than methamphetamine at reducing the visibility of the respective cells; the neurotoxicity of the compound involves activation of MAPK/ERK cascade and inhibition of Akt/PKB signaling pathway .[ 14] 25D-NBOMe , reduced the visibility of cardiomyocytes H9c2 cells, and both substances downregulated expression level of p21 (CDC24/RAC)-activated kinase 1 (PAK1), an enzyme with documented cardiac protective effects.[ 14]
Preliminary studies on 25C-NBOMe have shown that the substance is toxic to development, heart health, and brain health in
zebrafish , rats, and
Artemia salina , a common organism for studying potential drug effects on humans, but more research is needed on the topic, the dosages, and if the toxicology results apply to humans. Researchers of the study also recommended further investigation of the drug's potential in damaging pregnant women and their fetus due to the substance's damaging effects to development.
[ 32] [ 33]
Emergency treatment
Interactions
Pharmacology
Pharmacodynamics
25C-NBOMe activities
Target Affinity (Ki , nM)
5-HT1A 2,353–5,000
5-HT1B 2,372
5-HT1D 1,024
5-HT1E 5,776
5-HT1F ND
5-HT2A 0.7–1.6 (Ki )EC50 Tooltip half-maximal effective concentration )Emax Tooltip maximal efficacy )
5-HT2B 1.1 (Ki )EC50 )Emax )
5-HT2C 5.2–5.4 (Ki )EC50 )Emax )
5-HT3 >10,000
5-HT4 ND
5-HT5A 4,796
5-HT6 36.2
5-HT7 1,729
α1A 810–2,319
α1B >10,000
α1D >10,000
α2A 560–3,175
α2B 224
α2C 185
β1 –β3 ND
D1 12,000
D2 1,600–7,508
D3 878–3,500
D4 >10,000
D5 >10,000
H1 90
H2 –H4 ND
M1 –M3 >10,000
M4 5,410
M5 >10,000
I1 ND
σ1 441
σ2 41
MOR ND (Ki )EC50 )Emax )
DOR ND
KOR ND
TAAR1 Tooltip Trace amine-associated receptor 1 15,000 (Ki ) (mouse)i ) (rat)EC50 ) (mouse)EC50 ) (rat)EC50 ) (human)Emax ) (mouse)Emax ) (rat)
SERT Tooltip Serotonin transporter 1,500–>10,000 (Ki )IC50 Tooltip half-maximal inhibitory concentration )ND (EC50 )
NET Tooltip Norepinephrine transporter 1,600–>10,000 (Ki )IC50 )ND (EC50 )
DAT Tooltip Dopamine transporter 14,000 (Ki )IC50 )ND (EC50 )
Notes: The smaller the value, the more avidly the drug binds to the site. All proteins are human unless otherwise specified. Refs: [ 34] [ 5] [ 35] [ 36] [ 37] [ 38] [ 39]
25C-NBOMe acts as a serotonin 5-HT2 receptor agonist , including of the serotonin 5-HT2A receptor .
Chemistry
25C-NBOMe is derived from the psychedelic phenethylamine 2C-C by substitution on the amine with a 2-methoxybenzyl group. 25C-NBOMe is a clumpy white powder with a notably bitter and metallic taste.[ 41]
Analogues and derivatives
History
25C-NBOMe was first described in the scientific literature by Anders Ettrup and colleagues by 2010.[ 7] [ 5]
Society and culture
Recreational use
25C-NBOMe has been found on blotter mimics sold as LSD.[ 41]
Legal status
Canada
As of October 31, 2016; 25C-NBOMe is a controlled substance (Schedule III) in Canada.[ 43]
China
As of October 2015, 25C-NBOMe is a controlled substance in China.[ 44]
Czech Republic
25C-NBOMe is banned in the Czech Republic.[ 45]
Israel
The NBOMe series of psychoactives became controlled in Israel in May, 2013.[ 46] [ 47]
New Zealand
25C-NBOMe was sold as a designer drug in New Zealand in early 2012, but was withdrawn from sale after a statement by Associate Health Minister Peter Dunne that 25C-NBOMe would be considered to be substantially similar in chemical structure to the illegal hallucinogen DOB , and was therefore a Class C controlled drug analogue.[ 48]
Russia
Russia became the first country to regulate the NBOME class. The entire NBOMe series of psychoactives became controlled in the Russian Federation starting October, 2011.[ 46] [ 49]
Sweden
Sveriges riksdag "substances, plant materials and fungi which normally do not have medical use" ) as narcotics in Sweden as of Aug 1, 2013, published by Medical Products Agency [ 50]
United Kingdom
This substance is a Class A drug in the United Kingdom as a result of the N -benzylphenethylamine catch-all clause in the Misuse of Drugs Act 1971 .[ 51]
United States
Several NBOMe series compounds will be temporarily scheduled in the United States for 2 years. The temporary scheduling applies to 25C-NBOMe, 25B-NBOMe, and 25I-NBOMe.[ 52] [ 53]
Notes
^ The potency of N -benzylphenethylamines via buccal, sublingual, or nasal absorption is 50- to 100-fold greater (by weight) than oral route compared to the parent 2C-x compounds.[ 24] N -benzylphenethylamines is likely causing the low bioavailability on the oral route, although the metabolic profile of this compounds remains unpredictable; therefore researchers state that the fatalities linked to these substances may partly be explained by differences in the metabolism between individuals.[ 24]
References
^ Anvisa (2023-07-24). "RDC Nº 804 - Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial" [Collegiate Board Resolution No. 804 - Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control] (in Brazilian Portuguese). Diário Oficial da União (published 2023-07-25). Archived from the original on 2023-08-27. Retrieved 2023-08-27 .^ "Substance Details 25C-NBOMe" . Retrieved 2024-01-23 .^ Jolanta Z, Monika K, and Piotr A (26 February 2020). "NBOMes–Highly Potent and Toxic Alternatives of LSD" . Frontiers in Neuroscience . 14 78. doi :10.3389/fnins.2020.00078 PMC 7054380 PMID 32174803 . ^ Hansen M, Phonekeo K, Paine JS, Leth-Petersen S, Begtrup M, Bräuner-Osborne H, Kristensen JL (March 2014). "Synthesis and structure-activity relationships of N-benzyl phenethylamines as 5-HT2A/2C agonists" . ACS Chemical Neuroscience . 5 (3): 243– 249. doi :10.1021/cn400216u . PMC 3963123 PMID 24397362 . ^ a b c d Ettrup A, Hansen M, Santini MA, Paine J, Gillings N, Palner M, et al. (April 2011). "Radiosynthesis and in vivo evaluation of a series of substituted 11C-phenethylamines as 5-HT (2A) agonist PET tracers". European Journal of Nuclear Medicine and Molecular Imaging . 38 (4): 681– 693. doi :10.1007/s00259-010-1686-8 . PMID 21174090 . S2CID 12467684 . ^ Hansen M (2010-12-16). Design and Synthesis of Selective Serotonin Receptor Agonists for Positron Emission Tomography Imaging of the Brain (Ph.D. thesis). University of Copenhagen. doi :10.13140/RG.2.2.33671.14245 . ^ a b Ettrup, A. (2010). Serotonin receptor studies in the pig brain: pharmacological intervention and positron emission tomography tracer development (Doctoral dissertation, Faculty of Health Sciences, University of Copenhagen). https://research.regionh.dk/en/publications/serotonin-receptor-studies-in-the-pig-brain-pharmacological-inter
^ Kamińska K, Świt P, Malek K (2020). "25C-NBOMe short characterisation" . Forensic Toxicology . 38 (2): 490– 495. doi :10.1007/s11419-020-00530-1 S2CID 214704393 . ^ Lützen E, Holtkamp M, Stamme I, Schmid R, Sperling M, Pütz M, Karst U (April 2020). "Multimodal imaging of hallucinogens 25C- and 25I-NBOMe on blotter papers" . Drug Testing and Analysis . 12 (4): 465– 471. doi :10.1002/dta.2751 PMID 31846172 . S2CID 209388281 . ^ 2C-C-NBOMe Dose - erowid ^ Grautoff S, Kähler J (May 2014). "[Near fatal intoxication with the novel psychoactive substance 25C-NBOMe]". Medizinische Klinik, Intensivmedizin und Notfallmedizin (in German). 109 (4): 271– 275. doi :10.1007/s00063-014-0360-5 . PMID 24770890 . ^ a b c Sean I, Joe R, Jennifer S, and Shaun G (28 March 2022). "A cluster of 25B-NBOH poisonings following exposure to powder sold as lysergic acid diethylamide (LSD)" Clinical Toxicology 60 (8): 966– 969. doi :10.1080/15563650.2022.2053150 . PMID 35343858 . S2CID 247764056 . ^ a b c d Amy E, Katherine W, John R, Sonyoung K, Robert J, Aaron J (December 2018). "Neurochemical pharmacology of psychoactive substituted N-benzylphenethylamines: High potency agonists at 5-HT2A receptors" . Biochemical Pharmacology . 158 : 27– 34. doi :10.1016/j.bcp.2018.09.024 . PMC 6298744 PMID 30261175 . ^ a b c d e Jolanta Z, Monika K, and Piotr A (26 February 2020). "NBOMes–Highly Potent and Toxic Alternatives of LSD" . Frontiers in Neuroscience . 14 78. doi :10.3389/fnins.2020.00078 PMC 7054380 PMID 32174803 . Recently, a new class of psychedelic compounds named NBOMe (or 25X-NBOMe) has appeared on the illegal drug market. NBOMes are analogs of the 2C family of phenethylamine drugs, originally synthesized by Alexander Shulgin, that contain a N-(2-methoxy)benzyl substituent. The most frequently reported drugs from this group are 25I-NBOMe, 25B-NBOMe, and 25C-NBOMe. NBOMe compounds are ultrapotent and highly efficacious agonists of serotonin 5-HT2A and 5-HT2C receptors (Ki values in low nanomolar range) with more than 1000-fold selectivity for 5-HT2A compared with 5-HT1A. They display higher affinity for 5-HT2A receptors than their 2C counterparts and have markedly lower affinity, potency, and efficacy at the 5-HT2B receptor compared to 5-HT2A or 5-HT2C. ^ a b c d e f Lipow M, Kaleem SZ, Espiridion E (30 March 2022). "NBOMe Toxicity and Fatalities: A Review of the Literature" . Transformative Medicine . 1 (1): 12– 18. doi :10.54299/tmed/msot8578 ISSN 2831-8978 . S2CID 247888583 . ^ Tirri M, Bilel S, Arfè R, Corli G, Marchetti B, Bernardi T, Boccuto F, Serpelloni G, Botrè F, De-Giorgio F, Golembiowska K, Marti M (2022). "Effect of -NBOMe Compounds on Sensorimotor, Motor, and Prepulse Inhibition Responses in Mice in Comparison With the 2C Analogs and Lysergic Acid Diethylamide: From Preclinical Evidence to Forensic Implication in Driving Under the Influence of Drugs" . Front Psychiatry . 13 875722. doi :10.3389/fpsyt.2022.875722 PMC 9069068 PMID 35530025 . ^ a b Cristina M, Matteo M, Nicholas P, Maria C, Micaela T, Raffaella A, Maria L (12 December 2019). "Neurochemical and Behavioral Profiling in Male and Female Rats of the Psychedelic Agent 25I-NBOMe" . Frontiers in Pharmacology . 10 1406. doi :10.3389/fphar.2019.01406 PMC 6921684 PMID 31915427 . ^ a b c Anna R, Dino L, Julia R, Daniele B, Marius H, Matthias L (December 2015). "Receptor interaction profiles of novel N-2-methoxybenzyl (NBOMe) derivatives of 2,5-dimethoxy-substituted phenethylamines (2C drugs)" Neuropharmacology . 99 : 546– 553. doi :10.1016/j.neuropharm.2015.08.034 . ISSN 1873-7064 . PMID 26318099 . S2CID 10382311 . ^ a b David W, Roumen S, Andrew C, Paul D (6 February 2015). "Prevalence of use and acute toxicity associated with the use of NBOMe drugs" Clinical Toxicology . 53 (2): 85– 92. doi :10.3109/15563650.2015.1004179 . PMID 25658166 . S2CID 25752763 . ^ Humston C, Miketic R, Moon K, Ma P, Tobias J (2017-06-05). "Toxic Leukoencephalopathy in a Teenager Caused by the Recreational Ingestion of 25I-NBOMe: A Case Report and Review of Literature" . Journal of Medical Cases . 8 (6): 174– 179. doi :10.14740/jmc2811w ISSN 1923-4163 . ^ Justin P, Stephen R, Kylin A, Alphonse P, Michelle P (2015). "Analysis of 25I-NBOMe, 25B-NBOMe, 25C-NBOMe and Other Dimethoxyphenyl-N-[(2-Methoxyphenyl) Methyl]Ethanamine Derivatives on Blotter Paper" . Journal of Analytical Toxicology . 39 (8): 617– 623. doi :10.1093/jat/bkv073 . PMC 4570937 PMID 26378135 . ^ Morini L, Bernini M, Vezzoli S, Restori M, Moretti M, Crenna S, Papa P, Locatelli C, Osculati AM, Vignali C, Groppi A (October 2017). "Death after 25C-NBOMe and 25H-NBOMe consumption" Forensic Science International . 279 : e1 – e6 . doi :10.1016/j.forsciint.2017.08.028 . PMID 28893436 . ^ Byard RW, Cox M, Stockham P (November 2016). "Blunt Craniofacial Trauma as a Manifestation of Excited Delirium Caused by New Psychoactive Substances" Journal of Forensic Sciences . 61 (6): 1546– 1548. doi :10.1111/1556-4029.13212 . PMID 27723094 . S2CID 4734566 . ^ a b Sabastian LP, Christoffer B, Martin H, Martin AC, Jan K, Jesper LK (14 February 2014). "Correlating the Metabolic Stability of Psychedelic 5-HT2A Agonists with Anecdotal Reports of Human Oral Bioavailability" Neurochemical Research . 39 (10): 2018– 2023. doi :10.1007/s11064-014-1253-y . PMID 24519542 . S2CID 254857910 . ^ Adam H (18 January 2017). "Pharmacology and Toxicology of N-Benzylphenethylamine ("NBOMe") Hallucinogens" . Neuropharmacology of New Psychoactive Substances . Current Topics in Behavioral Neurosciences. Vol. 32. Springer. pp. 283– 311. doi :10.1007/7854_2016_64 . ISBN 978-3-319-52444-3 PMID 28097528 . ^ Boris D, Cristian C, Marcelo K, Edwar F, Bruce KC (August 2016). "Analysis of 25 C NBOMe in Seized Blotters by HPTLC and GC–MS" . Journal of Chromatographic Science . 54 (7): 1153– 1158. doi :10.1093/chromsci/bmw095 . PMC 4941995 PMID 27406128 . ^ Bersani FS, Corazza O, Albano G, Valeriani G, Santacroce R, Bolzan Mariotti Posocco F, Cinosi E, Simonato P, Martinotti G, Bersani G, Schifano F (2014). "25C-NBOMe: preliminary data on pharmacology, psychoactive effects, and toxicity of a new potent and dangerous hallucinogenic drug" . Biomed Res Int . 2014 734749. doi :10.1155/2014/734749 PMC 4106087 PMID 25105138 . ^ Adam JP, Simon HT, Simon LH (September 2021). "Pharmacology and toxicology of N-Benzyl-phenylethylamines (25X-NBOMe) hallucinogens" . Novel Psychoactive Substances: Classification, Pharmacology and Toxicology (2 ed.). Academic Press. pp. 279– 300. doi :10.1016/B978-0-12-818788-3.00008-5 . ISBN 978-0-12-818788-3 S2CID 240583877 . ^ Rothman RB, Baumann MH, Savage JE, Rauser L, McBride A, Hufeisen SJ, Roth BL (Dec 2000). "Evidence for possible involvement of 5-HT(2B) receptors in the cardiac valvulopathy associated with fenfluramine and other serotonergic medications" . Circulation . 102 (23): 2836– 41. doi :10.1161/01.CIR.102.23.2836 PMID 11104741 . ^ Fitzgerald LW, Burn TC, Brown BS, Patterson JP, Corjay MH, Valentine PA, Sun JH, Link JR, Abbaszade I, Hollis JM, Largent BL, Hartig PR, Hollis GF, Meunier PC, Robichaud AJ, Robertson DW (Jan 2000). "Possible role of valvular serotonin 5-HT(2B) receptors in the cardiopathy associated with fenfluramine". Molecular Pharmacology . 57 (1): 75– 81. doi :10.1016/S0026-895X(24)26444-0 . PMID 10617681 . ^ Roth BL (Jan 2007). "Drugs and valvular heart disease" The New England Journal of Medicine . 356 (1): 6– 9. doi :10.1056/NEJMp068265 . PMID 17202450 . ^ Xu P, Qiu Q, Li H, Yan S, Yang M, Naman CB, Wang Y, Zhou W, Shen H, Cui W (26 February 2019). "25C-NBOMe, a Novel Designer Psychedelic, Induces Neurotoxicity 50 Times More Potent Than Methamphetamine In Vitro" Neurotoxicity Research . 35 (4): 993– 998. doi :10.1007/s12640-019-0012-x . PMID 30806983 . S2CID 255763701 . ^ Álvarez-Alarcón N, Osorio-Méndez JJ, Ayala-Fajardo A, Garzón-Méndez WF, Garavito-Aguilar ZV (2021). "Zebrafish and Artemia salina in vivo evaluation of the recreational 25C-NBOMe drug demonstrates its high toxicity" . Toxicology Reports . 8 : 315– 323. Bibcode :2021ToxR....8..315A . doi :10.1016/j.toxrep.2021.01.010 . ISSN 2214-7500 . PMC 7868744 PMID 33598409 . ^ "Kᵢ Database" . PDSP . 9 June 2025. Retrieved 9 June 2025 .^ Hansen, M. (2010). Design and Synthesis of Selective Serotonin Receptor Agonists for Positron Emission Tomography Imaging of the Brain: PhD Thesis. Faculty of Pharmaceutical Sciences, University of Copenhagen. https://bitnest.netfirms.com/external/Theses/Hansen2011
^ Hansen M, Phonekeo K, Paine JS, Leth-Petersen S, Begtrup M, Bräuner-Osborne H, Kristensen JL (March 2014). "Synthesis and structure-activity relationships of N-benzyl phenethylamines as 5-HT2A/2C agonists" . ACS Chem Neurosci . 5 (3): 243– 249. doi :10.1021/cn400216u . PMC 3963123 PMID 24397362 . ^ Rickli A, Luethi D, Reinisch J, Buchy D, Hoener MC, Liechti ME (December 2015). "Receptor interaction profiles of novel N-2-methoxybenzyl (NBOMe) derivatives of 2,5-dimethoxy-substituted phenethylamines (2C drugs)" (PDF) . Neuropharmacology . 99 : 546– 553. doi :10.1016/j.neuropharm.2015.08.034 . PMID 26318099 . ^ Pottie E, Cannaert A, Stove CP (October 2020). "In vitro structure-activity relationship determination of 30 psychedelic new psychoactive substances by means of β-arrestin 2 recruitment to the serotonin 2A receptor". Arch Toxicol . 94 (10): 3449– 3460. Bibcode :2020ArTox..94.3449P . doi :10.1007/s00204-020-02836-w . PMID 32627074 . ^ Simmler LD, Buchy D, Chaboz S, Hoener MC, Liechti ME (April 2016). "In Vitro Characterization of Psychoactive Substances at Rat, Mouse, and Human Trace Amine-Associated Receptor 1" (PDF) . J Pharmacol Exp Ther . 357 (1): 134– 144. doi :10.1124/jpet.115.229765 . PMID 26791601 . Archived from the original (PDF) on 2025-05-09. ^ a b Zuba D, Sekuła K, Buczek A (April 2013). "25C-NBOMe--new potent hallucinogenic substance identified on the drug market". Forensic Science International . 227 (1– 3): 7– 14. doi :10.1016/j.forsciint.2012.08.027 . PMID 22989597 . ^ "Explore N-(2C-C)-Fentanyl | PiHKAL · info" . isomerdesign.com .^ "Regulations Amending the Food and Drug Regulations (Part J — 2C-phenethylamines)" . Government of Canada . 4 May 2016. Archived from the original on 5 August 2022. Retrieved 6 May 2023 .^ "关于印发《非药用类麻醉药品和精神药品列管办法》的通知" (in Chinese). China Food and Drug Administration. 27 September 2015. Archived from the original on 1 October 2015. Retrieved 1 October 2015 .^ "Látky, o které byl doplněn seznam č. 4 psychotropních látek (příloha č. 4 k nařízení vlády č. 463/2013 Sb.)" (PDF) (in Czech). Ministerstvo zdravotnictví. Archived from the original (PDF) on 2016-03-09. Retrieved 2016-02-06 .^ a b "NBOMe Series Legal Status" . Erowid. Retrieved 5 September 2015 .^ "Amendment to Dangerous Drugs Ordinance" . Israeli Ministry of Health . 7 June 2013. Retrieved 11 September 2015 .^ 'Legal high' DIME not so legal. Science Media Centre, March 13th 2012 ^ "Постановление Правительства Российской Федерации от 6 октября 2011 г. N 822 г. Москва" (in Russian). 19 October 2011. Retrieved 5 September 2015 .^ Åkerman CR (24 July 2013). "Föreskrifter om ändring i Läkemedelsverkets föreskrifter (LVFS 2011:10) om förteckningar över narkotika" (PDF) . Retrieved 5 September 2015 . ^ "The Misuse of Drugs Act 1971 (Ketamine etc.) (Amendment) Order 2014" . UK Statutory Instruments 2014 No. 1106 . www.legislation.gov.uk.^ Harrigan TM (10 October 2013). "Proposed Rules" (PDF) . Drug Enforcement Administration (DEA). Retrieved 5 September 2015 . ^ Drug Enforcement Administration (November 2015). "Schedules of Controlled Substances: Extension of Temporary Placement of Three Synthetic Phenethylamines in Schedule I. Final order". Federal Register . 80 (219): 70657– 70659. PMID 26567439 .
External links
No ring subs. 4-Hydroxytryptamines 5-Hydroxytryptamines 5-Methoxytryptamines Other ring subs.
2,N ,N -TMT 4,N ,N -TMT 5-Bromo-DMT 5-Chloro-DMT 5-Fluoro-DMT 5-N ,N -TMT 7,N ,N -TMT 5-MeO-2,N ,N -TMT 5-MeO-4,N ,N -TMT 6-Fluoro-DMT Bretisilocin (GM-2505; 5-fluoro-MET) α-Alkyltryptamines
5-Methoxy-α-alkyltryptamines: 5-MeO-AET α,N ,N -TMT (α-Me-DMT; Alpha-N) 5-MeO-AMT (α,O -DMS; Alpha-O) α,N ,O -TMS (5-MeO-α,N -DMT) α,N ,N ,O -TeMS (5-MeO-α,N ,N -TMT) Others
Ergolines /lysergamides (e.g., LSD )β-Carbolines and Harmala alkaloidsharmine , harmaline , 6-methoxyharmalan )Iboga alkaloids18-MAC , 18-MC , coronaridine , ibogaine , ibogamine , ME-18-MC , noribogaine , tabernanthine , voacangine )Ibogalogs (e.g., ibogainalog )O -MethylnordehydrobufoteninePartial ergolines (e.g., NDTDI , RU-28306 , CT-5252 )Piperidinylethylindoles (e.g., pip-T )Pyrrolidinylethylindoles (e.g., pyr-T , 5-MeO-pyr-T )Pyrrolidinylmethylindoles (e.g., MPMI , 4-HO-MPMI (lucigenol) , 5-MeO-MPMI )
Benzofurans (e.g., 5-MeO-DiBF , dimemebfe (5-MeO-BFE) , mebfap )Benzothiophenes (e.g., 3-APBT )Indazolethylamines (e.g., AL-38022A , O -methyl-AL-34662Indenylethylamines (e.g., C-DMT )Isotryptamines (e.g., 6-MeO-isoDMT , Ro60-0175 )MYCO-005 Quinolinylethylamines (e.g., mefloquine )
Others: 2C-G-x (e.g., 2C-G-3 , 2C-G-5 )β-Keto-2C-B (βk-2C-B) β-Keto-2C-I (βk-2C-I) β-Methyl-2C-B (BMB) BOB , BOD , BOH-2C-B )HOT-2 , HOT-7 , HOT-17 )N -Ethyl-2C-B2CD-2-ETO , 2CD-5-ETO , 2CE-5-ETO , 2CE-5iPrO , 2CT2-5-ETO , ASR-2001 (2CB-5PrO) ) Others
2-TOET 2-TOM 25B-NAcPip 4-HA 5-TOET 5-TOM Benzofurans (e.g., 5-APB , 5-APDB , 6-APB , 6-APDB , F , F-2 , F-22 )Benzothiophenes (e.g., 5-APBT , 6-APBT )CT-5172 DMAs (e.g., 2,4-DMA , 3,4-DMA )Fenfluramine MMA (3-MeO-4-MA) Norfenfluramine 25D-NM-NDEAOP , DOB-NDEPA , DOI-NDEPA , DOM-NDEPA , DOTFM-NDEPA , M-NDEPA , TMA-2-NDEPA )PMA (4-MA) TMA-3 , TMA-4 , TMA-5 )TOMSO ZDCM-04
1-Aminomethylindanes (e.g., 2CB-Ind , jimscaline )2-Aminoindanes (e.g., DOM-AI )3-Phenylpiperidines (e.g., LPH-5 , LPH-48 )Benzazepines (e.g., lorcaserin )Benzocyclobutenes (e.g., 2CBCB-NBOMe , TCB-2 , tomscaline )Benzoxepins (e.g., BBOX , IBOX , TFMBOX )DMBMPP (juncosamine) Ergolines /lysergamides (e.g., LSD )Glaucine Partial ergolines (e.g., NDTDI , DEIMDHPCA , DEMPDHPCA , DEMTMPDHPCA , DEMNDHPCA )Phenylcyclopropylamines (e.g., DMCPA , TMT )Phenyloxazolamines (aminorexes ) (e.g., 2C-B-aminorex )Pyridopyrroloquinoxalines (e.g., IHCH-7113 )Z3517967757 ZC-B
Others
Arylpiperazines (e.g., 2C-B-PP , 2-NP , mCPP , MK-212 , ORG-12962 , pCPP , pFPP , quipazine , TFMPP )Dihydrobenzoxazines (e.g., efavirenz )Phenoxyethylamines (e.g., CT-4719 , ORG-37684 )Pyridopyrroloquinoxalines (e.g., IHCH-7113 )Quinazolinylethylamines (e.g., RH-34 ) Natural sources
Tryptamines: Acacia spp.Acacia acuminata Acacia confusa Ayahuasca and vinho de Jurema (e.g., Psychotria viridis (chacruna)Dipolopterys cabrerana (chaliponga, chacruna)Mimosa tenuiflora (Mimosa hostilis ; jurema)Brosimum Brosimum acutifolium (takini)Hallucinogenic snuffs (e.g., Anadenanthera peregrina (yopo, jopo, cohoba, parica, ebene)Anadenanthera colubrina (vilca, cebil)Incilius alvarius (Bufo alvarius ; Colorado River toad, Sonoran Desert toad; bufo)Psilocybin-containing mushrooms (magic mushrooms, shrooms) (e.g., Psilocybe cubensis Psilocybe mexicana (teonanacatl)Lysergamides: Achnatherum robustum (sleepy grass)Epichloë spp.Ergot (Claviceps ) (e.g., Claviceps purpurea Claviceps paspali Morning glory (Convolvulaceae) seeds (e.g., Ipomoea tricolor (tlitliltzin, badoh negro; Ipomoea violacea )Ipomoea corymbosa (coaxihuitl, ololiúqui; Rivea Corymbosa , Turbina Corymbosa )Argyreia nervosa (Hawaiian baby woodrose; HBWR)Periglandula spp.Periglandula ipomoeae Periglandula clandestina
5-HT1
5-HT1A
Agonists: 8-OH-DPAT Adatanserin Amphetamine Antidepressants (e.g., etoperidone , hydroxynefazodone , nefazodone , trazodone , triazoledione , vilazodone , vortioxetine )Atypical antipsychotics (e.g., aripiprazole , asenapine , brexpiprazole , cariprazine , clozapine , lurasidone , quetiapine , ziprasidone )Azapirones (e.g., buspirone , eptapirone , gepirone , perospirone , tandospirone )Bay R 1531 Befiradol BMY-14802 Cannabidiol Dimemebfe Dopamine Ebalzotan Eltoprazine Enciprazine Ergolines (e.g., bromocriptine , cabergoline , dihydroergotamine , ergotamine , lisuride , LSD , methylergometrine (methylergonovine) , methysergide , pergolide )F-11461 F-12826 F-13714 F-14679 F-15063 F-15599 Flesinoxan Flibanserin Flumexadol Hypidone Lesopitron LY-293284 LY-301317 mCPP MKC-242 Naluzotan NBUMP Osemozotan Oxaflozane Pardoprunox Piclozotan Rauwolscine Repinotan Roxindole RU-24969 S-14506 S-14671 S-15535 Sarizotan Serotonin (5-HT) SSR-181507 Sunepitron Tryptamines (e.g., 5-CT , 5-MeO-DMT , 5-MT , bufotenin , DMT , indorenate , N-Me-5-HT , psilocin , psilocybin )TGBA01AD U-92016-A Urapidil Vilazodone Xaliproden Yohimbine
Positive allosteric modulators: Oleamide
Antagonists: Atypical antipsychotics (e.g., iloperidone , risperidone , sertindole )AV965 Beta blockers (e.g., alprenolol , carteolol , cyanopindolol , iodocyanopindolol , isamoltane , oxprenolol , penbutolol , pindobind , pindolol , propranolol , tertatolol )BMY-7378 CSP-2503 Dotarizine Ergolines (e.g., metergoline )FCE-24379 Flopropione GR-46611 Isamoltane Lecozotan Mefway Metitepine (methiothepin) MIN-117 (WF-516) MPPF NAN-190 Robalzotan S-15535 SB-649915 SDZ 216-525 Spiperone Spiramide Spiroxatrine UH-301 WAY-100135 WAY-100635 Xylamidine
5-HT1B
Agonists: Anpirtoline CGS-12066A CP-93129 CP-94253 CP-122288 CP-135807 Eltoprazine Ergolines (e.g., bromocriptine , dihydroergotamine , ergotamine , methylergometrine (methylergonovine) , methysergide , pergolide )mCPP RU-24969 Serotonin (5-HT) Triptans (e.g., avitriptan , donitriptan , eletriptan , sumatriptan , zolmitriptan )TFMPP Tryptamines (e.g., 5-BT , 5-CT , 5-MT , DMT )Vortioxetine
5-HT1D
Agonists: CP-122288 CP-135807 CP-286601 Ergolines (e.g., bromocriptine , cabergoline , dihydroergotamine , ergotamine , LSD , methysergide )GR-46611 L-694247 L-772405 mCPP PNU-109291 PNU-142633 Serotonin (5-HT) TGBA01AD Triptans (e.g., almotriptan , avitriptan , donitriptan , eletriptan , frovatriptan , naratriptan , rizatriptan , sumatriptan , zolmitriptan )Tryptamines (e.g., 5-BT , 5-CT , 5-Et-DMT , 5-MT , 5-(nonyloxy)tryptamine , DMT )
5-HT1E
5-HT1F
5-HT2
5-HT2A
Agonists: 25H/NB series (e.g., 25I-NBF , 25I-NBMD , 25I-NBOH , 25I-NBOMe , 25B-NBOMe , 25C-NBOMe , 25TFM-NBOMe , 2CBCB-NBOMe , 25CN-NBOH , 2CBFly-NBOMe )2Cs (e.g., 2C-B , 2C-E , 2C-I , 2C-T-2 , 2C-T-7 , 2C-T-21 )2C-B-FLY 2CB-Ind 5-Methoxytryptamines (5-MeO-DET , 5-MeO-DiPT , 5-MeO-DMT , 5-MeO-DPT , 5-MT )α-Alkyltryptamines (e.g., 5-Cl-αMT , 5-Fl-αMT , 5-MeO-αET , 5-MeO-αMT , α-Me-5-HT , αET , αMT )AL-34662 AL-37350A Bromo-DragonFLY Dimemebfe DMBMPP DOx (e.g., DOB , DOC , DOI , DOM )Efavirenz Ergolines (e.g., 1P-LSD , ALD-52 , bromocriptine , cabergoline , ergine (LSA) , ergometrine (ergonovine) , ergotamine , lisuride , LA-SS-Az , LSB , LSD , LSD-Pip , LSH , LSP , methylergometrine (methylergonovine) , pergolide )Flumexadol IHCH-7113 Jimscaline Lorcaserin MDxx (e.g., MDA (tenamfetamine) , MDMA (midomafetamine) , MDOH , MMDA )O-4310 Oxaflozane PHA-57378 PNU-22394 PNU-181731 RH-34 SCHEMBL5334361 Phenethylamines (e.g., lophophine , mescaline )Piperazines (e.g., BZP , quipazine , TFMPP )Serotonin (5-HT) TCB-2 TFMFly Tryptamines (e.g., 5-BT , 5-CT , bufotenin , DET , DiPT , DMT , DPT , psilocin , psilocybin , tryptamine )
Antagonists: 5-I-R91150 5-MeO-NBpBrT AC-90179 Adatanserin Altanserin Antihistamines (e.g., cyproheptadine , hydroxyzine , ketotifen , perlapine )AMDA Atypical antipsychotics (e.g., amperozide , aripiprazole , asenapine , blonanserin , brexpiprazole , carpipramine , clocapramine , clorotepine , clozapine , fluperlapine , gevotroline , iloperidone , lurasidone , melperone , mosapramine , ocaperidone , olanzapine , paliperidone , quetiapine , risperidone , sertindole , zicronapine , ziprasidone , zotepine )Chlorprothixene Cinanserin CSP-2503 Deramciclane Dotarizine Eplivanserin Ergolines (e.g., amesergide , LY-53857 , LY-215840 , mesulergine , metergoline , methysergide , sergolexole )Fananserin Flibanserin Glemanserin Irindalone Ketanserin KML-010 Landipirdine LY-393558 mCPP Medifoxamine Metitepine (methiothepin) MIN-117 (WF-516) Naftidrofuryl Nantenine Nelotanserin Opiranserin (VVZ-149) Pelanserin Phenoxybenzamine Pimavanserin Pirenperone Pizotifen Pruvanserin Rauwolscine Ritanserin Roluperidone S-14671 Sarpogrelate Serotonin antagonists and reuptake inhibitors (e.g., etoperidone , hydroxynefazodone , lubazodone , mepiprazole , nefazodone , triazoledione , trazodone )SR-46349B TGBA01AD Teniloxazine Temanogrel Tetracyclic antidepressants (e.g., amoxapine , aptazapine , esmirtazapine , maprotiline , mianserin , mirtazapine )Tricyclic antidepressants (e.g., amitriptyline )Typical antipsychotics (e.g., chlorpromazine , fluphenazine , haloperidol , loxapine , perphenazine , pimozide , pipamperone , prochlorperazine , setoperone , spiperone , spiramide , thioridazine , thiothixene , trifluoperazine )Volinanserin Xylamidine Yohimbine
5-HT2B
Agonists: 4-Methylaminorex Aminorex Amphetamines (e.g., chlorphentermine , cloforex , dexfenfluramine , fenfluramine , levofenfluramine , norfenfluramine )BW-723C86 DOx (e.g., DOB , DOC , DOI , DOM )Ergolines (e.g., cabergoline , dihydroergocryptine , dihydroergotamine , ergotamine , methylergometrine (methylergonovine) , methysergide , pergolide )Lorcaserin MDxx (e.g., MDA (tenamfetamine) , MDMA (midomafetamine) , MDOH , MMDA )Piperazines (e.g., TFMPP )PNU-22394 Ro60-0175 Serotonin (5-HT) Tryptamines (e.g., 5-BT , 5-CT , 5-MT , α-Me-5-HT , bufotenin , DET , DiPT , DMT , DPT , psilocin , psilocybin , tryptamine )
Antagonists: Agomelatine Atypical antipsychotics (e.g., amisulpride , aripiprazole , asenapine , brexpiprazole , cariprazine , clozapine , N-desalkylquetiapine (norquetiapine) , N-desmethylclozapine (norclozapine) , olanzapine , pipamperone , quetiapine , risperidone , ziprasidone )Cyproheptadine EGIS-7625 Ergolines (e.g., amesergide , bromocriptine , lisuride , LY-53857 , LY-272015 , mesulergine )Ketanserin LY-393558 mCPP Metadoxine Metitepine (methiothepin) Pirenperone Pizotifen Propranolol PRX-08066 Rauwolscine Ritanserin RS-127445 Sarpogrelate SB-200646 SB-204741 SB-206553 SB-215505 SB-221284 SB-228357 SDZ SER-082 Tegaserod Tetracyclic antidepressants (e.g., amoxapine , mianserin , mirtazapine )Trazodone Typical antipsychotics (e.g., chlorpromazine )TIK-301 Yohimbine
5-HT2C
Agonists: 2Cs (e.g., 2C-B , 2C-E , 2C-I , 2C-T-2 , 2C-T-7 , 2C-T-21 )5-Methoxytryptamines (5-MeO-DET , 5-MeO-DiPT , 5-MeO-DMT , 5-MeO-DPT , 5-MT )α-Alkyltryptamines (e.g., 5-Cl-αMT , 5-Fl-αMT , 5-MeO-αET , 5-MeO-αMT , α-Me-5-HT , αET , αMT )A-372159 AL-38022A Alstonine CP-809101 Dimemebfe DOx (e.g., DOB , DOC , DOI , DOM )Ergolines (e.g., ALD-52 , cabergoline , dihydroergotamine , ergine (LSA) , ergotamine , lisuride , LA-SS-Az , LSB , LSD , LSD-Pip , LSH , LSP , pergolide )Flumexadol Lorcaserin MDxx (e.g., MDA (tenamfetamine) , MDMA (midomafetamine) , MDOH , MMDA )MK-212 ORG-12962 ORG-37684 Oxaflozane PHA-57378 Phenethylamines (e.g., lophophine , mescaline )Piperazines (e.g., aripiprazole , BZP , mCPP , quipazine , TFMPP )PNU-22394 PNU-181731 Ro60-0175 Ro60-0213 Serotonin (5-HT) Tryptamines (e.g., 5-BT , 5-CT , bufotenin , DET , DiPT , DMT , DPT , psilocin , psilocybin , tryptamine )Vabicaserin WAY-629 WAY-161503 YM-348
Antagonists: Adatanserin Agomelatine Atypical antipsychotics (e.g., asenapine , clorotepine , clozapine , fluperlapine , iloperidone , melperone , olanzapine , paliperidone , quetiapine , risperidone , sertindole , ziprasidone , zotepine )Captodiame CEPC Cinanserin Cyproheptadine Deramciclane Desmetramadol Dotarizine Eltoprazine Ergolines (e.g., amesergide , bromocriptine , LY-53857 , LY-215840 , mesulergine , metergoline , methysergide , sergolexole )Etoperidone Fluoxetine FR-260010 Irindalone Ketanserin Ketotifen Latrepirdine (dimebolin) Medifoxamine Metitepine (methiothepin) Nefazodone Pirenperone Pizotifen Propranolol Ritanserin RS-102221 S-14671 SB-200646 SB-206553 SB-221284 SB-228357 SB-242084 SB-243213 SDZ SER-082 Tedatioxetine Tetracyclic antidepressants (e.g., amoxapine , aptazapine , esmirtazapine , maprotiline , mianserin , mirtazapine )TIK-301 Tramadol Trazodone Tricyclic antidepressants (e.g., amitriptyline , nortriptyline )Typical antipsychotics (e.g., chlorpromazine , loxapine , pimozide , pipamperone , thioridazine )Xylamidine
5-HT3 –7
5-HT3
Agonists: Alcohols (e.g., butanol , ethanol (alcohol) , trichloroethanol )m-CPBG Phenylbiguanide Piperazines (e.g., BZP , mCPP , quipazine )RS-56812 Serotonin (5-HT) SR-57227 SR-57227A Tryptamines (e.g., 2-Me-5-HT , 5-CT , bufotenidine (5-HTQ) )Volatiles/gases (e.g., halothane , isoflurane , toluene , trichloroethane )YM-31636
Antagonists: Alosetron Anpirtoline Arazasetron AS-8112 Atypical antipsychotics (e.g., clozapine , olanzapine , quetiapine )Azasetron Batanopride Bemesetron (MDL-72222) Cilansetron CSP-2503 Dazopride Dolasetron Galanolactone Granisetron Lerisetron Memantine Ondansetron Palonosetron Ramosetron Renzapride Ricasetron Tedatioxetine Tetracyclic antidepressants (e.g., amoxapine , mianserin , mirtazapine )Thujone Tropanserin Tropisetron Typical antipsychotics (e.g., loxapine )Volatiles/gases (e.g., nitrous oxide , sevoflurane , xenon )Vortioxetine Zacopride Zatosetron
5-HT4
5-HT5A
5-HT6
Agonists: Ergolines (e.g., dihydroergocryptine , dihydroergotamine , ergotamine , lisuride , LSD , mesulergine , metergoline , methysergide )Hypidone Serotonin (5-HT) Tryptamines (e.g., 2-Me-5-HT , 5-BT , 5-CT , 5-MT , Bufotenin , E-6801 , E-6837 , EMD-386088 , EMDT , LY-586713 , N-Me-5-HT , ST-1936 , tryptamine )WAY-181187 WAY-208466
Antagonists: ABT-354 Atypical antipsychotics (e.g., aripiprazole , asenapine , clorotepine , clozapine , fluperlapine , iloperidone , olanzapine , tiospirone )AVN-101 AVN-211 AVN-322 AVN-397 BGC20-760 BVT-5182 BVT-74316 Cerlapirdine EGIS-12233 GW-742457 Idalopirdine Ketanserin Landipirdine Latrepirdine (dimebolin) Masupirdine Metitepine (methiothepin) MS-245 PRX-07034 Ritanserin Ro 04-6790 Ro 63-0563 SB-258585 SB-271046 SB-357134 SB-399885 SB-742457 Tetracyclic antidepressants (e.g., amoxapine , mianserin )Tricyclic antidepressants (e.g., amitriptyline , clomipramine , doxepin , nortriptyline )Typical antipsychotics (e.g., chlorpromazine , loxapine )
5-HT7
Antagonists: Atypical antipsychotics (e.g., amisulpride , aripiprazole , asenapine , brexpiprazole , clorotepine , clozapine , fluperlapine , olanzapine , risperidone , sertindole , tiospirone , ziprasidone , zotepine )Butaclamol DR-4485 EGIS-12233 Ergolines (e.g., 2-Br-LSD (BOL-148) , amesergide , bromocriptine , cabergoline , dihydroergotamine , ergotamine , LY-53857 , LY-215840 , mesulergine , metergoline , methysergide , sergolexole )JNJ-18038683 Ketanserin LY-215840 Metitepine (methiothepin) Ritanserin SB-258719 SB-258741 SB-269970 SB-656104 SB-656104A SB-691673 SLV-313 SLV-314 Spiperone SSR-181507 Tetracyclic antidepressants (e.g., amoxapine , maprotiline , mianserin , mirtazapine )Tricyclic antidepressants (e.g., amitriptyline , clomipramine , imipramine )Typical antipsychotics (e.g., acetophenazine , chlorpromazine , chlorprothixene , fluphenazine , loxapine , pimozide )Vortioxetine
Negative allosteric modulators: Oleamide
Phenethylamines Amphetamines Phentermines Cathinones Phenylisobutylamines (and further-extended) Catecholamines (and close relatives) Cyclized
Phenylalkylpyrrolidines 2-Benzylpiperidines (phenidates ) Phenylmorpholines (phenmetrazines) Phenyloxazolamines (aminorexes) Isoquinolines andtetrahydroisoquinolines 2-Aminoindanes 2-Aminotetralins Others / unsorted
1-Aminomethylindanes (e.g., 2CB-Ind , AMMI , bromojimscaline , jimscaline )2-ADN 2-Benzhydrylpyrrolidine 2C-B-5-hemiFLY-α6 (BNAP) 2C-B-PYR 2CBecca 2CJP 2CLisaB 2CLisaH 3-Benzhydrylmorpholine 3-Phenylpiperidines (e.g., 3-phenylpiperidine , 3-PPP , OSU-6162 (PNU-96391) , LPH-5 , LPH-48 , Z3517967757 (Z7757) )6-AB AL-1095 Aminochromes (e.g., adrenochrome , adrenolutin )Benzazepines (e.g., fenoldopam , lorcaserin , SCHEMBL5334361 )Benzocyclobutenes (e.g., 2CBCB-NBOMe , bromotomscaline , S33005 , TCB-2 , tomscaline )Benzoxepins (e.g., BBOX , IBOX , TFMBOX )Butyltolylquinuclidine Camfetamine Cypenamine (trans -2-phenylcyclopentylamine) Diphenidine Diphenylprolinol DMBMPP Ergolines (e.g., LSD )Fencamfamin GYKI-52895 HDMP-29 Ivabradine Methoxphenidine Methylmorphenate Milnacipran MT-45 2-Naphthylamine Org 6582 Partial ergolines (e.g., NDTDI , RU-27849 , DEIMDHPCA , DEMPDHPCA , DEMPDHPCA-2C-D , RU-27251 )PF-592,379 Phenylcyclopropylamines (e.g., DMCPA , TMT , tranylcypromine )Phenylpiracetams (e.g., phenylpiracetam , MRZ-9547 , RGPU-95 )Pyridopyrroloquinoxalines (e.g., lumateperone , deulumateperone , IHCH-7079 , IHCH-7086 , IHCH-7113 , ITI-1549 )Tetrahydrobenzopyranylamines (e.g., CT-5126 )Tolazoline Tricyclics (e.g., AMDA , AMDH , benzoctamine , dizocilpine , SpAMDA )ZC-B
Related compounds
2-Furylethylamine 2-Pyrrolylethylamine 3-Pyrrolylethylamine 3-Pyrrolylpropylamine 2-Tetrahydrofurylethylamine 4-Benzylpiperidine 7-AB Alkylamines (e.g., 1,3-DMBA Tooltip 1,3-dimethylbutylamine , 1,4-DMAA Tooltip 1,4-dimethylamylamine , heptaminol , iproheptine , isometheptene , methylhexanamine/1,3-DMAA , octodrine , oenethyl , tuaminoheptane )Benzylamines (e.g., benzylamine , α-methylbenzylamine , MDM1EA , ALPHA , M-ALPHA , pargyline )Benzylpiperazines (e.g., benzylpiperazine , MDBZP , fipexide )Cyclohexylaminopropanes (e.g., propylhexedrine , norpropylhexedrine )Cyclopentylaminopropanes (e.g., isocyclamine , cyclopentamine )Phenoxyethylamines (e.g., 3,4,5-trimethoxyphenoxyethylamine , CT-4719 , ORG-37684 )Phenylalkenylamines (e.g., phenylbutenamine )Phenylalkynylamines (e.g., phenylbutynamine )Phenylpiperazines (e.g., 1-phenylpiperazine , mCPP Tooltip meta-chlorophenylpiperazine , TFMPP Tooltip trifluoromethylphenylpiperazine , oMPP Tooltip ortho-methylphenylpiperazine , pFPP Tooltip para-fluorophenylpiperazine , pMeOPP Tooltip para-methoxyphenylpiperazine )Phenylpropylamines (e.g., phenylpropylamine , homo-MDA , homo-MDMA )Thienylaminopropanes (thiopropamines) (e.g., thiopropamine , methiopropamine , thiothinone )