Staphylococcus aureus
  Staphylococcus aureus is a Gram-positive spherically shaped bacterium, a member of the Bacillota, and is a usual member of the microbiota of the body, frequently found in the upper respiratory tract and on the skin. It is often positive for catalase and nitrate reduction and is a facultative anaerobe, meaning that it can grow without oxygen.[1] Although S. aureus usually acts as a commensal of the human microbiota, it can also become an opportunistic pathogen, being a common cause of skin infections including abscesses, respiratory infections such as sinusitis, and food poisoning. Pathogenic strains often promote infections by producing virulence factors such as potent protein toxins, and the expression of a cell-surface protein that binds and inactivates antibodies. S. aureus is one of the leading pathogens for deaths associated with antimicrobial resistance and the emergence of antibiotic-resistant strains, such as methicillin-resistant S. aureus (MRSA). The bacterium is a worldwide problem in clinical medicine. Despite much research and development, no vaccine for S. aureus has been approved. An estimated 21% to 30% of the human population are long-term carriers of S. aureus,[2][3] which can be found as part of the normal skin microbiota, in the nostrils,[2][4] and as a normal inhabitant of the lower reproductive tract of females.[5][6] S. aureus can cause a range of illnesses, from minor skin infections, such as pimples,[7] impetigo, boils, cellulitis, folliculitis, carbuncles, scalded skin syndrome, and abscesses, to life-threatening diseases such as pneumonia, meningitis, osteomyelitis, endocarditis, toxic shock syndrome, bacteremia, and sepsis. It is still one of the five most common causes of hospital-acquired infections and is often the cause of wound infections following surgery. Each year, around 500,000 hospital patients in the United States contract a staphylococcal infection, chiefly by S. aureus.[8] Up to 50,000 deaths each year in the U.S. are linked to staphylococcal infection.[9] HistoryDiscoveryIn 1880, Alexander Ogston, a Scottish surgeon, discovered that Staphylococcus can cause wound infections after noticing groups of bacteria in pus from a surgical abscess during a procedure he was performing. He named it Staphylococcus after its clustered appearance evident under a microscope. Then, in 1884, German scientist Friedrich Julius Rosenbach identified Staphylococcus aureus, discriminating and separating it from Staphylococcus albus, a related bacterium. In the early 1930s, doctors began to use a more streamlined test to detect the presence of an S. aureus infection by the means of coagulase testing, which enables detection of an enzyme produced by the bacterium. Prior to the 1940s, S. aureus infections were fatal in the majority of patients. However, doctors discovered that the use of penicillin could cure S. aureus infections. Unfortunately, by the end of the 1940s, penicillin resistance became widespread amongst this bacterium population and outbreaks of the resistant strain began to occur.[10] EvolutionStaphylococcus aureus can be sorted into ten dominant human lineages.[11] There are numerous minor lineages as well, but these are not seen in the population as often. Genomes of bacteria within the same lineage are mostly conserved, with the exception of mobile genetic elements. Mobile genetic elements that are common in S. aureus include bacteriophages, pathogenicity islands, plasmids, transposons, and staphylococcal cassette chromosomes. These elements have enabled S. aureus to continually evolve and gain new traits. There is a great deal of genetic variation within the S. aureus species. A study by Fitzgerald et al. (2001) revealed that approximately 22% of the S. aureus genome is non-coding and thus can differ from bacterium to bacterium. An example of this difference is seen in the species' virulence. Only a few strains of S. aureus are associated with infections in humans. This demonstrates that there is a large range of infectious ability within the species.[12] It has been proposed that one possible reason for the great deal of heterogeneity within the species could be due to its reliance on heterogeneous infections. This occurs when multiple different types of S. aureus cause an infection within a host. The different strains can secrete different enzymes or bring different antibiotic resistances to the group, increasing its pathogenic ability.[13] Thus, there is a need for a large number of mutations and acquisitions of mobile genetic elements.[citation needed] Another notable evolutionary process within the S. aureus species is its co-evolution with its human hosts. Over time, this parasitic relationship has led to the bacterium's ability to be carried in the nasopharynx of humans without causing symptoms or infection. This allows it to be passed throughout the human population, increasing its fitness as a species.[14] However, only approximately 50% of the human population are carriers of S. aureus, with 20% as continuous carriers and 30% as intermittent. This leads scientists to believe that there are many factors that determine whether S. aureus is carried asymptomatically in humans, including factors that are specific to an individual person. According to a 1995 study by Hofman et al., these factors may include age, sex, diabetes, and smoking. They also determined some genetic variations in humans that lead to an increased ability for S. aureus to colonize, notably a polymorphism in the glucocorticoid receptor gene that results in larger corticosteroid production. In conclusion, there is evidence that any strain of this bacterium can become invasive, as this is highly dependent upon human factors.[15] Though S. aureus has quick reproductive and micro-evolutionary rates, there are multiple barriers that prevent evolution with the species. One such barrier is AGR, which is a global accessory gene regulator within the bacteria. This such regulator has been linked to the virulence level of the bacteria. Loss of function mutations within this gene have been found to increase the fitness of the bacterium containing it. Thus, S. aureus must make a trade-off to increase their success as a species, exchanging reduced virulence for increased drug resistance. Another barrier to evolution is the Sau1 Type I restriction modification (RM) system. This system exists to protect the bacterium from foreign DNA by digesting it. Exchange of DNA between the same lineage is not blocked, since they have the same enzymes and the RM system does not recognize the new DNA as foreign, but transfer between different lineages is blocked.[13] Microbiology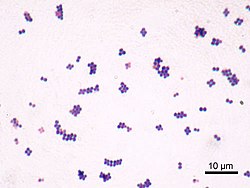  Staphylococcus aureus (/ˌstæfɪləˈkɒkəs ˈɔːriəs, -loʊ-/,[16][17] Greek σταφυλόκοκκος 'grape-cluster berry', Latin aureus, 'golden') is a facultative anaerobic, Gram-positive coccal (round) bacterium also known as "golden staph" and "oro staphira". S. aureus is nonmotile and does not form spores.[18] In medical literature, the bacterium is often referred to as S. aureus, Staph aureus or Staph a..[19] S. aureus appears as staphylococci (grape-like clusters) when viewed through a microscope, and has large, round, golden-yellow colonies, often with hemolysis, when grown on blood agar plates.[20] S. aureus reproduces asexually by binary fission. Complete separation of the daughter cells is mediated by S. aureus autolysin, and in its absence or targeted inhibition, the daughter cells remain attached to one another and appear as clusters.[21] Staphylococcus aureus is catalase-positive (meaning it can produce the enzyme catalase). Catalase converts hydrogen peroxide (H Natural genetic transformation is a reproductive process involving DNA transfer from one bacterium to another through the intervening medium, and the integration of the donor sequence into the recipient genome by homologous recombination. S. aureus was found to be capable of natural genetic transformation, but only at low frequency under the experimental conditions employed.[24] Further studies suggested that the development of competence for natural genetic transformation may be substantially higher under appropriate conditions, yet to be discovered.[25] Role in healthIn humans, S. aureus can be present in the upper respiratory tract, gut mucosa, and skin as a member of the normal microbiota.[26][27][28] However, because S. aureus can cause disease under certain host and environmental conditions, it is characterized as a pathobiont.[26] In the United States, MRSA infections alone are estimated to cost the healthcare system over $3.2 billion annually.[29] These infections account for nearly 20,000 deaths each year in the U.S., exceeding those caused by HIV/AIDS, Parkinson's disease, and homicide.[30] Annually, over 119,000 bloodstream infections in the U.S. are attributed to S. aureus.[31] S. aureus infections are ranked as one of the costliest healthcare-associated infections (HAIs), with each case averaging $23,000 to $46,000 in treatment and hospital resource utilization.[32] On average, patients with MRSA infections experience a lengthened hospital stay of approximately 6 to 11 days, which drives up inpatient care costs.[33][34] The burden extends beyond direct healthcare expenses. Indirect costs, such as lost wages, reduced productivity, and long-term disability, can significantly amplify the overall economic toll. Severe S. aureus infections, including bacteremia, endocarditis, and osteomyelitis, often require prolonged recovery and rehabilitation, affecting patients' ability to return to work or perform daily activities.[35] Hospitals also invest heavily in infection control protocols to limit the spread of S. aureus, especially drug-resistant strains. These measures include routine screening, isolation practices, use of personal protective equipment, and antibiotic stewardship programs, which collectively contribute to rising operational costs. These necessary preventative measures can raise hospital costs by tens of thousands of dollars.[36] Role in disease 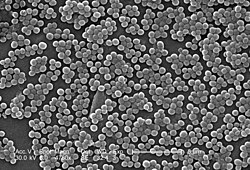 While S. aureus usually acts as a commensal bacterium, asymptomatically colonizing about 30% of the human population, it can sometimes cause disease.[3] In particular, S. aureus is one of the most common causes of bacteremia and infective endocarditis. Additionally, it can cause various skin and soft-tissue infections,[3] particularly when skin or mucosal barriers have been breached. Staphylococcus aureus infections can spread through contact with pus from an infected wound, skin-to-skin contact with an infected person, and contact with objects used by an infected person such as towels, sheets, clothing, or athletic equipment. Joint replacements put a person at particular risk of septic arthritis, staphylococcal endocarditis (infection of the heart valves), and pneumonia.[37] Staphylococcus aureus is a significant cause of chronic biofilm infections on medical implants, and the repressor of toxins is part of the infection pathway.[38] Staphylococcus aureus can lie dormant in the body for years undetected. Once symptoms begin to show, the host is contagious for another two weeks, and the overall illness lasts a few weeks. If untreated, though, the disease can be deadly.[39] Deeply penetrating S. aureus infections can be severe.[citation needed] Skin infectionsSkin infections are the most common form of S. aureus infection. This can manifest in various ways, including small benign boils, folliculitis, impetigo, cellulitis, and more severe, invasive soft-tissue infections.[7][3] Staphylococcus aureus is extremely prevalent in persons with atopic dermatitis (AD), more commonly known as eczema.[40] It is mostly found in fertile, active places, including the armpits, hair, and scalp. Large pimples that appear in those areas may exacerbate the infection if lacerated. Colonization of S. aureus drives inflammation of AD.[41][40] S. aureus is believed to exploit defects in the skin barrier of persons with atopic dermatitis, triggering cytokine expression and therefore exacerbating symptoms.[42] This can lead to staphylococcal scalded skin syndrome, a severe form of which can be seen in newborns.[43] The role of S. aureus in causing itching in atopic dermatitis has been studied.[44] Antibiotics are commonly used to target overgrowth of S. aureus but their benefit is limited and they increase the risk of antimicrobial resistance. For these reasons, they are only recommended for people who not only present symptoms on the skin but feel systematically unwell.[45][46][47] Food poisoningStaphylococcus aureus is also responsible for food poisoning and achieves this by generating toxins in the food, which is then ingested.[48] Its incubation period lasts 30 minutes to eight hours,[49] with the illness itself lasting from 30 minutes to 3 days.[50] Preventive measures one can take to help prevent the spread of the disease include washing hands thoroughly with soap and water before preparing food. The Centers for Disease Control and Prevention recommends staying away from any food if ill, and wearing gloves if any open wounds occur on hands or wrists while preparing food. If storing food for longer than 2 hours, it is recommended to keep the food below 4.4 or above 60 °C (below 40 or above 140 °F).[51] Bone and joint infectionsStaphylococcus aureus is a common cause of major bone and joint infections, including osteomyelitis, septic arthritis, and infections following joint replacement surgeries.[52][3][53] BacteremiaStaphylococcus aureus is a leading cause of bloodstream infections throughout much of the industrialized world.[52] Infection is generally associated with breaks in the skin or mucosal membranes due to surgery, injury, or use of intravascular devices such as cannulas, hemodialysis machines, or hypodermic needles.[3][52] Once the bacteria have entered the bloodstream, they can infect various organs, causing infective endocarditis, septic arthritis, and osteomyelitis.[52] This disease is particularly prevalent and severe in the very young and very old.[3] Without antibiotic treatment, S. aureus bacteremia has a case fatality rate around 80%.[3] With antibiotic treatment, case fatality rates range from 15% to 50% depending on the age and health of the patient, as well as the antibiotic resistance of the S. aureus strain.[3] Medical implant infectionsStaphylococcus aureus is often found in biofilms formed on medical devices implanted in the body or on human tissue. It is commonly found with another pathogen, Candida albicans, forming multispecies biofilms. The latter is suspected to help S. aureus penetrate human tissue.[9] A higher mortality is linked with multispecies biofilms.[54] Staphylococcus aureus biofilm is the predominant cause of orthopedic implant-related infections, but is also found on cardiac implants, vascular grafts, various catheters, and cosmetic surgical implants.[55][56] After implantation, the surface of these devices becomes coated with host proteins, which provide a rich surface for bacterial attachment and biofilm formation. Once the device becomes infected, it must be completely removed, since S. aureus biofilm cannot be destroyed by antibiotic treatments.[56] Current therapy for S. aureus biofilm-mediated infections involves surgical removal of the infected device followed by antibiotic treatment. Conventional antibiotic treatment alone is not effective in eradicating such infections.[55] An alternative to postsurgical antibiotic treatment is using antibiotic-loaded, dissolvable calcium sulfate beads, which are implanted with the medical device. These beads can release high doses of antibiotics at the desired site to prevent the initial infection.[56] Novel treatments for S. aureus biofilm involving nano silver particles, bacteriophages, and plant-derived antibiotic agents are being studied. These agents have shown inhibitory effects against S. aureus embedded in biofilms.[57] A class of enzymes have been found to have biofilm matrix-degrading ability, thus may be used as biofilm dispersal agents in combination with antibiotics.[58] Animal infectionsStaphylococcus aureus can survive on dogs,[59] cats,[60] and horses,[61] and can cause bumblefoot in chickens.[62] Some believe health-care workers' dogs should be considered a significant source of antibiotic-resistant S. aureus, especially in times of outbreak.[59] In a 2008 study by Boost, O'Donoghue, and James, it was found that just about 90% of S. aureus colonized within pet dogs presented as resistant to at least one antibiotic. The nasal region has been implicated as the most important site of transfer between dogs and humans.[63] Staphylococcus aureus is one of the causal agents of mastitis in dairy cows. Its large polysaccharide capsule protects the organism from recognition by the cow's immune defenses.[64] Virulence factorsEnzymesStaphylococcus aureus produces various enzymes such as coagulase (bound and free coagulases) which facilitates the conversion off fibrinogen to fibrin to cause clots which is important in skin infections.[65] Hyaluronidase (also known as spreading factor) breaks down hyaluronic acid and helps in spreading it. Deoxyribonuclease, which breaks down the DNA, protects S. aureus from neutrophil extracellular trap-mediated killing.[66][67] S. aureus also produces lipase to digest lipids, staphylokinase to dissolve fibrin and aid in spread, and beta-lactamase for drug resistance.[68] ToxinsDepending on the strain, S. aureus is capable of secreting several exotoxins, which can be categorized into three groups. Many of these toxins are associated with specific diseases.[69]
Type VII secretion systemA secretion system is a highly specialised multi-protein unit that is embedded in the cell envelope with the function of translocating effector proteins from inside of the cell to the extracellular space or into a target host cytosol. The exact structure and function of T7SS is yet to be fully elucidated. Currently, four proteins are known components of S. aureus type VII secretion system; EssC is a large integral membrane ATPase – which most likely powers the secretion systems and has been hypothesised forming part of the translocation channel. The other proteins are EsaA, EssB, EssA, that are membrane proteins that function alongside EssC to mediate protein secretion. The exact mechanism of how substrates reach the cell surface is unknown, as is the interaction of the three membrane proteins with each other and EssC.[76] T7 dependent effector proteins EsaD is DNA endonuclease toxin secreted by S. aureus, has been shown to inhibit growth of competitor S. aureus strain in vitro.[77] EsaD is cosecreted with chaperone EsaE, which stabilises EsaD structure and brings EsaD to EssC for secretion.[77][76] Strains that produce EsaD also co-produce EsaG, a cytoplasmic anti-toxin that protects the producer strain from EsaD's toxicity.[77] TspA is another toxin that mediates intraspecies competition. It is a bacteriostatic toxin that has a membrane depolarising activity facilitated by its C-terminal domain. Tsai is a transmembrane protein that confers immunity to the producer strain of TspA, as well as the attacked strains. There is genetic variability of the C-terminal domain of TspA therefore, it seems like the strains may produce different TspA variants to increase competitiveness.[78] Toxins that play a role in intraspecies competition confers an advantage by promoting successful colonisation in polymicrobial communities such as the nasopharynx and lung by outcompeting lesser strains.[78] There are also T7 effector proteins that play role a in pathogenesis, for example mutational studies of S. aureus have suggested that EsxB and EsxC contribute to persistent infection in a murine abscess model.[79] EsxX has been implicated in neutrophil lysis, therefore suggested as contributing to the evasion of host immune system. Deletion of essX in S. aureus resulted in significantly reduced resistance to neutrophils and reduced virulence in murine skin and blood infection models.[80] Altogether, T7SS and known secreted effector proteins are a strategy of pathogenesis by improving fitness against competitor S. aureus species as well as increased virulence via evading the innate immune system and optimising persistent infections.[citation needed] Small RNAThe list of small RNAs involved in the control of bacterial virulence in S. aureus is growing. This can be facilitated by factors such as increased biofilm formation in the presence of increased levels of such small RNAs.[81] For example, RNAIII,[82] SprD,[83] SprC,[84][85] RsaE,[86] SprA1,[87] SSR42,[88] ArtR,[89] SprX, Teg49,[81] and IsrR.[90] DNA repairHost neutrophils cause DNA double-strand breaks in S. aureus through the production of reactive oxygen species.[91] For infection of a host to be successful, S. aureus must survive such damages caused by the hosts' defenses. The two protein complex RexAB encoded by S. aureus is employed in the recombinational repair of DNA double-strand breaks.[91] Strategies for post-transcriptional regulation by 3'untranslated regionMany mRNAs in S. aureus carry three prime untranslated regions (3'UTR) longer than 100 nucleotides, which may potentially have a regulatory function.[92] Further investigation of icaR mRNA (mRNA coding for the repressor of the main expolysaccharidic compound of the bacteria biofilm matrix) demonstrated that the 3'UTR binding to the 5' UTR can interfere with the translation initiation complex and generate a double stranded substrate for RNase III. The interaction is between the UCCCCUG motif in the 3'UTR and the Shine-Dalagarno region at the 5'UTR. Deletion of the motif resulted in IcaR repressor accumulation and inhibition of biofilm development.[92] The biofilm formation is the main cause of Staphylococcus implant infections.[93] BiofilmBiofilms are groups of microorganisms, such as bacteria, that attach to each other and grow on wet surfaces.[94] The S. aureus biofilm is embedded in a glycocalyx slime layer and can consist of teichoic acids, host proteins, extracellular DNA (eDNA) and sometimes polysaccharide intercellular antigen (PIA). S. aureus biofilms are important in disease pathogenesis, as they can contribute to antibiotic resistance and immune system evasion.[56] S. aureus biofilm has high resistance to antibiotic treatments and host immune response.[94] One hypothesis for explaining this is that the biofilm matrix protects the embedded cells by acting as a barrier to prevent antibiotic penetration. However, the biofilm matrix is composed with many water channels, so this hypothesis is becoming increasingly less likely, but a biofilm matrix possibly contains antibiotic‐degrading enzymes such as β-lactamases, which can prevent antibiotic penetration.[95] Another hypothesis is that the conditions in the biofilm matrix favor the formation of persister cells, which are highly antibiotic-resistant, dormant bacterial cells.[56] S. aureus biofilms also have high resistance to host immune response. Though the exact mechanism of resistance is unknown, S. aureus biofilms have increased growth under the presence of cytokines produced by the host immune response.[96] Host antibodies are less effective for S. aureus biofilm due to the heterogeneous antigen distribution, where an antigen may be present in some areas of the biofilm, but completely absent from other areas.[56] Studies in biofilm development have shown to be related to changes in gene expression. There are specific genes that were found to be crucial in the different biofilm growth stages. Two of these genes include rocD and gudB, which encode for the enzyme's ornithine-oxo-acid transaminase and glutamate dehydrogenase, which are important for amino acid metabolism. Studies have shown biofilm development rely on amino acids glutamine and glutamate for proper metabolic functions.[97] Other immunoevasive strategies
Protein A is anchored to staphylococcal peptidoglycan pentaglycine bridges (chains of five glycine residues) by the transpeptidase sortase A.[98] Protein A, an IgG-binding protein, binds to the Fc region of an antibody. In fact, studies involving mutation of genes coding for protein A resulted in a lowered virulence of S. aureus as measured by survival in blood, which has led to speculation that protein A-contributed virulence requires binding of antibody Fc regions.[99] Protein A in various recombinant forms has been used for decades to bind and purify a wide range of antibodies by immunoaffinity chromatography. Transpeptidases, such as the sortases responsible for anchoring factors like protein A to the staphylococcal peptidoglycan, are being studied in hopes of developing new antibiotics to target MRSA infections.[100] 
Some strains of S. aureus are capable of producing staphyloxanthin – a golden-coloured carotenoid pigment. This pigment acts as a virulence factor, primarily by being a bacterial antioxidant which helps the microbe evade the reactive oxygen species which the host immune system uses to kill pathogens.[101][102] Mutant strains of S. aureus modified to lack staphyloxanthin are less likely to survive incubation with | ||||||||||||||||||||||||||||||||||||||||||||||
