Rhenium hexafluoride
Rhenium hexafluoride, also rhenium(VI) fluoride, (ReF6) is a compound of rhenium and fluorine and one of the seventeen known binary hexafluorides. ChemistryRhenium hexafluoride is made by combining rhenium heptafluoride with additional rhenium metal at 300 °C in a pressure vessel.[2]
The compound is a Lewis acid and strong oxidant, adducting potassium fluoride and oxidizing nitric oxide to nitrosyl:[3]
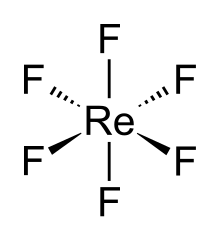
DescriptionRhenium hexafluoride is a liquid at room temperature. At 18.5 °C, it freezes into a yellow solid. The boiling point is 33.7 °C.[1] The solid structure measured at −140 °C is orthorhombic space group Pnma. Lattice parameters are a = 9.417 Å, b = 8.570 Å, and c = 4.965 Å. There are four formula units (in this case, discrete molecules) per unit cell, giving a density of 4.94 g·cm−3.[2] The ReF6 molecule itself (the form important for the liquid or gas phase) has octahedral molecular geometry, which has point group (Oh). The Re–F bond length is 1.823 Å.[2] UseRhenium hexafluoride is a commercial material used in the electronics industry for depositing films of rhenium.[3] References
Further reading
External links |
||||||||||||||||||||||||||||||||||||||||||||||