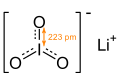Lithium iodate
Skeletal formula of lithium iodate with I—O bond length
Crystal structure of lithium iodate, iodines are inside the unit cell
Names
IUPAC name
Lithium iodate
Identifiers
ChemSpider
ECHA InfoCard 100.033.954
EC Number
UNII
UN number
1479
InChI=1S/HIO3.Li/c2-1(3)4;/h(H,2,3,4);/q;+1/p-1
Y Key: FZAXZVHFYFGNBX-UHFFFAOYSA-M
Y
Properties
LiIO3
Appearance
White hygroscopic crystals
Odor
Odorless
Density
4.487 g/cm3 [ 1]
Melting point
420–450 °C (788–842 °F; 693–723 K)[ 1] [ 3] [ 5]
Anhydrous:[ 1] [ 2]
Solubility
Insoluble in EtOH [ 3]
−47.0·10−6 cm3 /mol
Thermal conductivity
1.27 W/m·K (a-axis)[ 1]
1.8875 (20 °C)n He–Ne [ 1] [ 4]
Structure
Hexagonal ,[ 3] hP10 [ 6]
P63 22, No. 182[ 6]
622[ 6]
a = 5.46(9) Å,
c = 5.15(5) Å
[ 6] α = 90°, β = 90°, γ = 120°
Hazards
GHS labelling
[ 7]
Danger
H272 , H315 , H319 , H335 , H360 [ 7]
P201 , P220 , P261 , P305+P351+P338 , P308+P313 [ 7]
NFPA 704
Except where otherwise noted, data are given for materials in their
standard state (at 25 °C [77 °F], 100 kPa).
Chemical compound
Lithium iodate (LiIO3 ) is a negative uniaxial crystal[ 1] [ 9] [ 10]
Properties
Mohs hardness of lithium iodate is 3.5–4. Its linear thermal expansion coefficient at 298 K (25 °C; 77 °F) is 2.8·10−5 /°C (a-axis) and 4.8·10−5 /°C (c-axis).[ 1] [ 5]
References
^ a b c d e f g "Rarely Used and Archive Crystals". Nonlinear Optical Crystals: A Complete Survey 364– 368. doi :10.1007/0-387-27151-1_8 . ISBN 978-0-387-27151-4 the original on 2014-08-08. Retrieved 2014-08-08 . ^ Seidell, Atherton; Linke, William F. (1919). Solubilities of Inorganic and Organic Compounds New York City : D. Van Nostrand Company. p. 374 . ^ a b c Lide, David R., ed. (2009). CRC Handbook of Chemistry and Physics Boca Raton, Florida : CRC Press . ISBN 978-1-4200-9084-0 ^ Polyanskiy, Mikhail. "Refractive index of LiIO3 (Lithium iodate) - Herbst-o" . refractiveindex.info . Retrieved 2014-08-08 . ^ a b Teyssier, Jeremie; Dantec, Ronan Le; Galez, Christine; Mugnier, Yannick; Bouillot, Jacques; Plenet, Jean-Claude (2003-11-20). "LiIO 3 nanocrystals in SiO 2 xerogels, a new material for nonlinear optics" . In Andrews, David L; Gaburro, Zeno; Cartwright, Alexander N; Lee, Charles Y. C (eds.). Nanocrystals, and Organic and Hybrid Nanomaterials . Vol. 5222. p. 26. Bibcode :2003SPIE.5222...26T . CiteSeerX 10.1.1.605.1743 doi :10.1117/12.507309 . S2CID 136547473 . ^ a b c d Zachariasen, W.H.; Olof, F.A. BartaLars (1931-06-15). "Crystal Structure of Lithium Iodate". Physical Review Letters 37 (12): 1626– 1630. Bibcode :1931PhRv...37.1626Z . doi :10.1103/PhysRev.37.1626 . ^ a b c Sigma-Aldrich Co. , Lithium iodate . Retrieved on 2014-08-08.^ "SDS of Lithium iodate anhydrous" (PDF) . pfaltzandbauer.com . Connecticut , USA: Pfaltz & Bauer, Inc. Archived from the original (PDF) on 2014-08-10. Retrieved 2014-08-08 .^ Risk, W. P.; Gosnell, T. R.; Nurmikko, A. V. (9 January 2003). Compact Blue-Green Lasers Cambridge University Press . p. 123. ISBN 978-0-521-52103-1 . Retrieved 13 December 2012 . ^ Nikogosyan, David N. (4 January 2005). Nonlinear Optical Crystals: A Complete Survey ISBN 978-0-387-22022-2 . Retrieved 13 December 2012 .