Geological history of oxygen
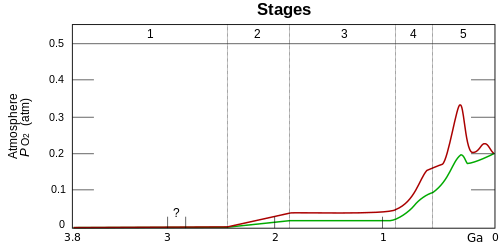 Stage 1 (3.85–2.45 Ga): Practically no O2 in the atmosphere. Stage 2 (2.45–1.85 Ga): O2 produced, but absorbed in oceans and seabed rock. Stage 3 (1.85–0.85 Ga): O2 starts to gas out of the oceans, but is absorbed by land surfaces and formation of ozone layer. Stages 4 and 5 (0.85 Ga–present): O2 sinks filled, the gas accumulates.[1] Although oxygen is the most abundant element in Earth's crust, due to its high reactivity it mostly exists in compound (oxide) forms such as water, carbon monoxide/dioxide, iron oxides and silicates. Before photosynthesis evolved, Earth's atmosphere had little free diatomic elemental oxygen (O2).[2] Small quantities of oxygen were released by geological[3] and biological processes, but did not build up in the reducing atmosphere due to reactions with then-abundant reducing gases such as atmospheric methane and hydrogen sulfide and surface reductants such as ferrous iron. Oxygen began building up in the prebiotic atmosphere at approximately 2.45 Ga during the Neoarchean-Paleoproterozoic boundary, a paleogeological event known as the Great Oxygenation Event (GOE). The concentrations of O2 attained were less than 10% of today's and probably fluctuated greatly. Around 500Mya a second event known as the Neoproterozoic Oxygenation Event lead to oxygen levels similar or even higher than the present. The increase in oxygen concentrations had wide-ranging and significant impacts on Earth's geochemistry and biosphere. Detailed connections between oxygen and evolution remain elusive. ImportanceOxygen is both a result of biological activity and a key enabler. Photosynthesis produces oxygen while plants and animals using aerobic respiration consume it. Consequently the evolution of life is closely related to the concentration of available oxygen. Understanding the relationship between oxygen and evolution would aid in seeking evidence of extraterrestrial life in exoplanet data.[4]: 252 Oxygen concentration plays a key role in the geochemical composition of sedimentary rocks, making oxygen concentration important for geology and sedimentary rocks important for understanding oxygen concentration over geologic time.[5] OverviewDue to limitations in measurements, oxygen concentration in the atmosphere or oceans over much of the early parts of Earth's history remains controversial.[6] The consensus view includes these phases:
The timing and character of the oxygenation events have been the subject of many discussions.[6] Paleo-oxybarometersTechniques for estimating oxygen at different times in the past are called "paleo-oxybarometers". An ideal technique would rely on trapped gas or fluid in a well-dated rock layer, but such examples are scarce. Most measurements are indirect analysis of oxidation in sedimentary rocks to infer oxygen in ancient atmosphere or oceans. Ocean analysis is especially challenging because tectonic subduction replaces the ocean floor every 200 million years. Many new techniques have been developed but their analysis and comparison have lead to additional debates rather than consensus on the oxygen history of Earth.[8] Prebiotic atmosphereEarth's early atmosphere had a very low concentration of oxygen, probably less than 0.001% of present day levels. While details are not well known, measurements of mass-independent fractionation of sulfur in sedimentary sulphides and sulfates rule out significant oxygen before around 2.45Gya.[1] Continuing sources of oxygen during this period would have been photodissociation of water followed by escape the hydrogen product[9] or of sulfur dioxide.[10] Great Oxygenation EventBetween 2.45 and 2 Gya, oxygen began to build up in the atmosphere. This time coincided with major changes in geochemistry and biology on the Earth but which changes are causes and which are results are debated.[1]: 905 Widespread production of oxygen by cyanobacteria which evolved around this time is suggestive, but substantial evidence suggests that cyanobacteria appeared at least 2.7Ga and perhaps well before that. Geological effects like volcanism, weathering, and burial of chemically altered rocks may be important.[11]: 6 Oxygen began to persist in the atmosphere in small quantities about 50 million years before the start of the Great Oxygenation Event.[12] By 800Mya oxygen in the atmosphere was between 1% and 18% of the present atmospheric level.[11] Neoproterozoic Oxygenation EventBy around 600Mya, during the Neoproterozoic, oxygen levels rose again, persisting throughout the Phanerozoic at near modern day values. During the Cambrian, atmospheric oxygen 5-10% concentration, around half its current value. It rose in pulses above 15% of the atmosphere during the Devonian Age of the Fishes", reaching 25% (above modern 20%) in the Permo-Carboniferous.[4] Evolution and oxygen
Atmospheric oxygen is the most conspicuous sign of life on Earth. The evolution of photosynthesis and the rise of oxygen-producing cyanobacteria are major events in evolution. Photosynthetic oxygen eventually accumulated in the atmosphere, transforming both the surface of the planet and the nature of life[11] Despite these connections, the details are not simple. The evolution of life and the geological history of oxygen share many similar patterns, but the relationship between these two histories remains uncertain. For example, the rise in oxygen concencentration and the rise the maximum size of organism have similar histories, but evidence the oxygen concentration limits size is inconclusive. As more evidence for lower and variable levels of oxygen before the Neoproterozoic has emerged, simple relationships between life and oxygen have been harder to justify.[13] See alsoReferences
External links
|
||||||||||||||||||||||||||||||||||||













