Duff reactionThe Duff reaction or hexamine aromatic formylation is a formylation reaction used in organic chemistry for the synthesis of benzaldehydes with hexamine as the formyl carbon source. The method is generally inefficient.[1] The reaction is named after James Cooper Duff.[2] The reaction requires strongly electron donating substituents on the aromatic ring such as in a phenol. Formylation occurs ortho to the electron donating substituent preferentially, unless the ortho positions are blocked, in which case the formylation occurs at the para position.[3] ExamplesThe modified salicylaldehyde 3,5-di-tert-butylsalicylaldehyde is prepared by the Duff reaction:[4] 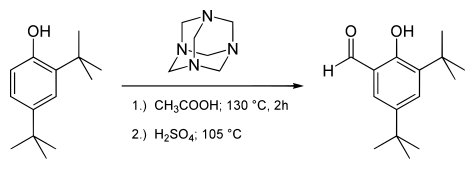 The natural product syringaldehyde can also be prepared by the Duff reaction. In this example, formylation occurs at the position para to the phenolic OH.[5]  Unlike other formylation reactions the Duff reaction is able to attach multiple aldehyde groups. If both ortho positions are vacant then a diformylation is possible, as in the formation of diformylcresol from p-cresol.[6] Conversion of phenol to the corresponding 1,3,5-trialdehyde has also been reported[7] Reaction mechanismThe reaction mechanism is related to that for the Reimer–Tiemann reaction, which uses chloroform as the formylating agent.[1] Protonated hexamine ring-opens to expose an iminium group. Addition to the aromatic ring results in an intermediate at the oxidation state of a benzylamine. An intramolecular redox reaction then ensues, raising the benzylic carbon to the oxidation state of an aldehyde. The oxygen atom is provided by water on acid hydrolysis in the final step.  Historical referencesDuff was a chemist at the College of Technology, Birmingham, around 1920–1950.[2] who
See also
References
|













